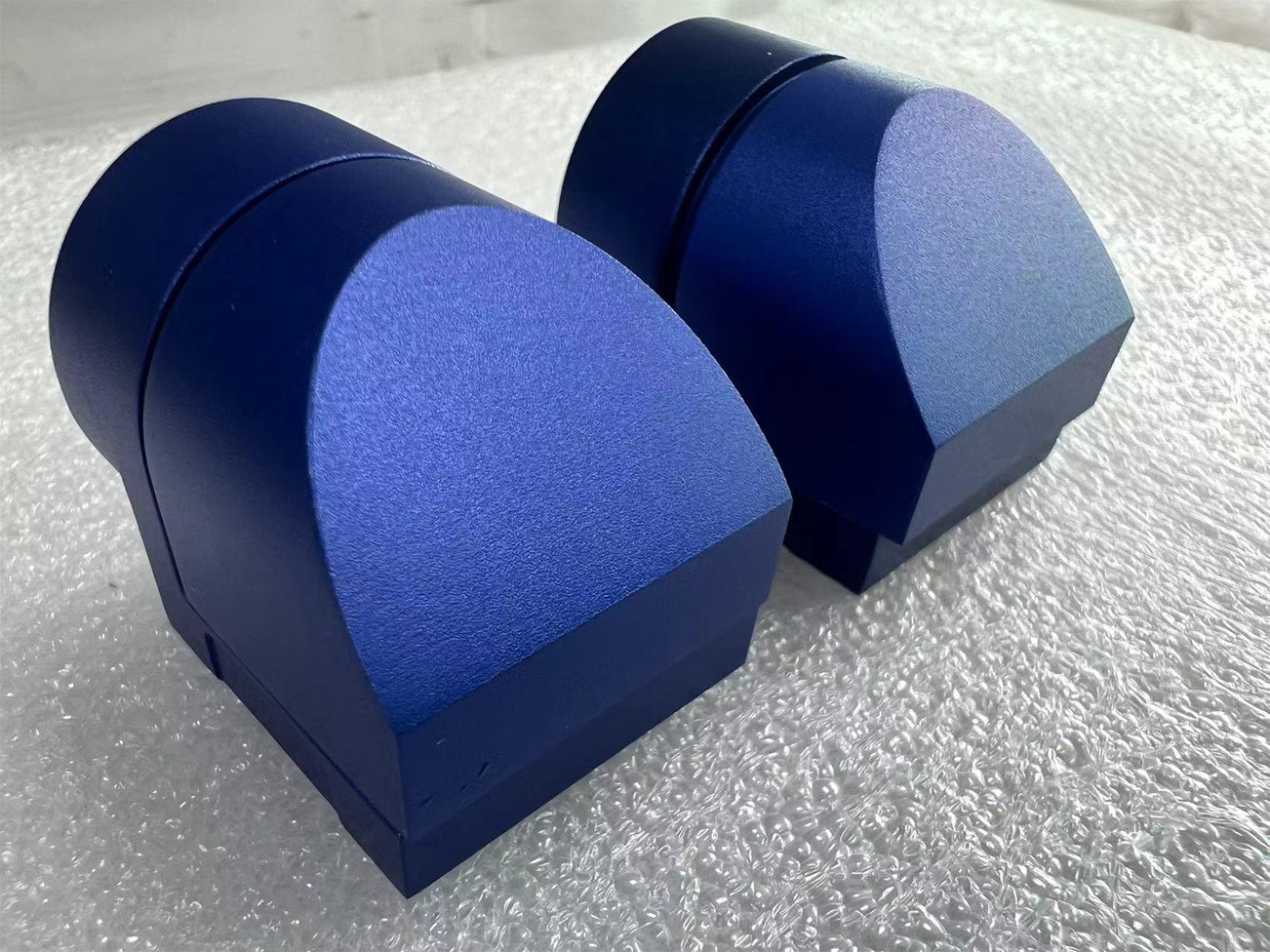
Anodizing
Anodized finish is a process used to coat metal surfaces with a protective oxide layer, typically aluminum, through a technique called anodizing.
During anodizing, the metal is immersed in an electrolyte solution and subjected to an electric current.
This process causes oxygen ions to bond with the metal surface, forming a durable and corrosion-resistant oxide layer.
Anodized finishes offer several advantages:
Enhanced Durability:
The anodized layer provides increased resistance to corrosion, abrasion, and wear, extending the lifespan of the metal component.
Color Options:
Anodizing can be performed in a variety of colors, allowing for customization and aesthetic enhancement of the metal surface.
Improved Adhesion:
The porous nature of the anodized layer promotes better adhesion of paints, adhesives, and other coatings, enhancing the overall finish and performance.
Electrical Insulation:
Anodized finishes provide electrical insulation, making them suitable for applications where conductivity needs to be minimized.
Environmental Friendliness:
Anodizing is an environmentally friendly process, as it does not involve the use of harsh chemicals or generate hazardous waste.
Overall, anodized finishes are widely used in industries such as aerospace, automotive, architecture, and consumer electronics, where durable and aesthetically pleasing metal surfaces are desired.
Production Process:
1. Mechanical polishing;
2. Chemical treatment to remove copper components from the surface of certain alloys;
3. Cleaning to remove oil (for parts that have already been anodized, if re-anodizing is needed, alkali or special agents are used to remove the original anodized surface layer);
4. Placed into dilute sulfuric acid as an anode for electrification, forming a surface oxide layer (which is porous, appearing as a white semi-transparent film);
5. Dyeing;
6. Fixation (heating or using chromate solution to seal the pores of the surface oxide layer).