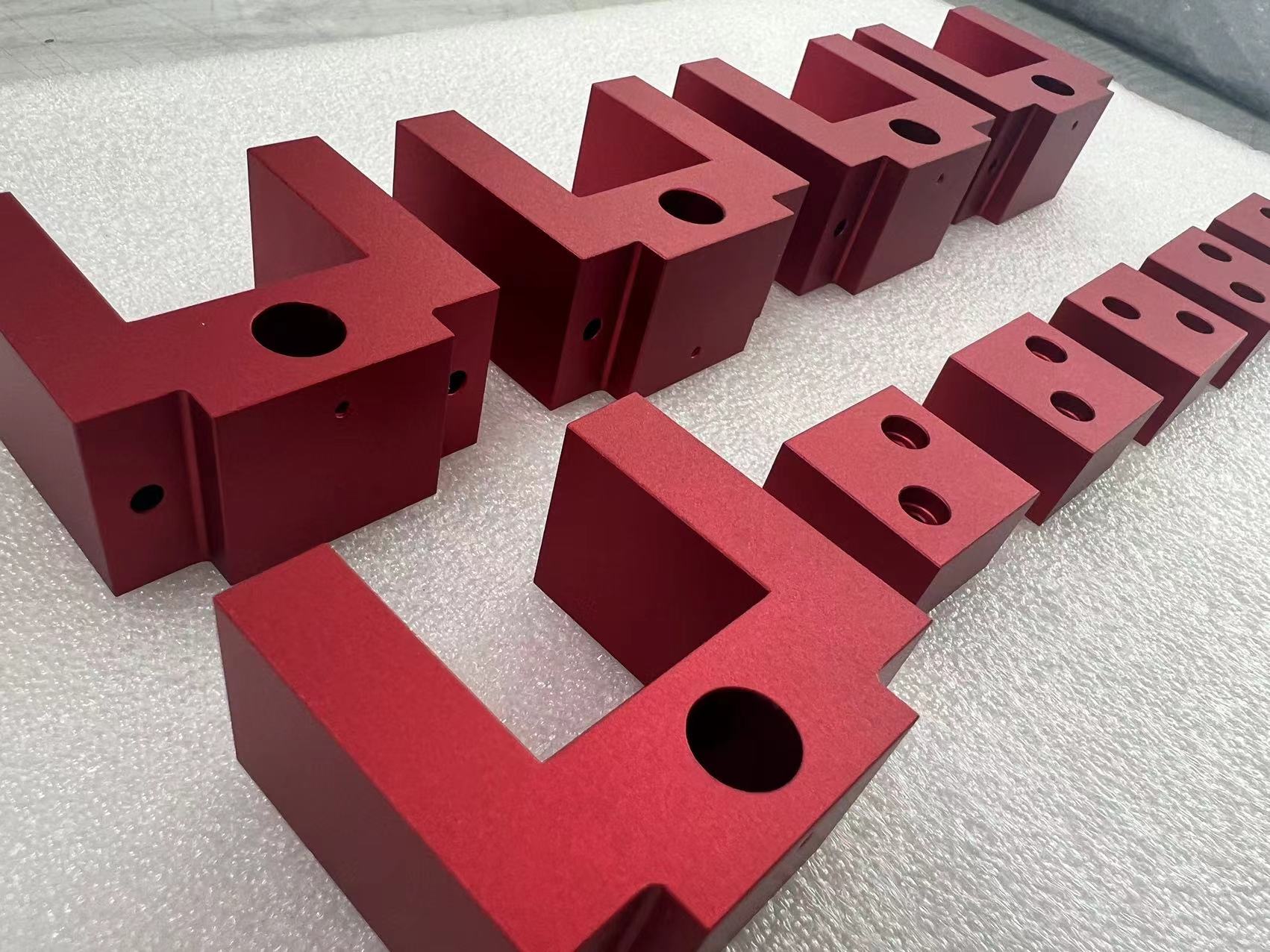
Aluminum alloy red anodized
Anodizing is the electrochemical oxidation of metals or alloys.
Aluminum and its alloys, under specific electrolyte and process conditions, undergo the formation of an oxide film on the aluminum product (anode) due to the application of external current.
Unless specified otherwise, anodizing typically refers to sulfuric acid anodizing.
To overcome deficiencies in surface hardness, wear resistance, and to extend lifespan, surface treatment technologies have become indispensable in the use of aluminum alloys, with anodizing being the most widely applied and successful technique.
Production Process:
1. Mechanical polishing;
2. Chemical treatment to remove copper components from certain alloy surfaces;
3. Degreasing (for parts already anodized, if re-anodizing is needed, alkaline or specialized agents are used to remove the original anodized surface layer);
4. Immersion in dilute sulfuric acid as the anode for electrification, resulting in the formation of a surface oxide layer (which is porous and appears as a white semi-transparent film);
5. Dyeing;
6. Sealing (heating or using chromate solutions to close the pores of the surface oxide layer).